1. Problems that you faced in the learning of Science and how you overcome these
problems?
Overall, I feel that there is no difficulty in learning science so long as you understand the concept. I tried various methods to understand science perhaps using a real life example to link the concept. In the case of the electronic structure, I used a Ferris wheel to represent the shell. Sometimes, I do dissect the definition of each word to understand it thoroughly though it may be time consuming.
---------------------------------------------------------------------------------------------------------------
2. What are the scientific concepts that I have learnt?
1) The Snell's Law which is used to measure the refractive index of an optical medium.
Snell's Law uses the equation:
It is the ratio of the angle of incidence to the angle of refraction where n is a constant.
For example:
This concept is very useful for measuring the refractive index of a material.
Note that the angle of incidence must be in the optical medium of air.
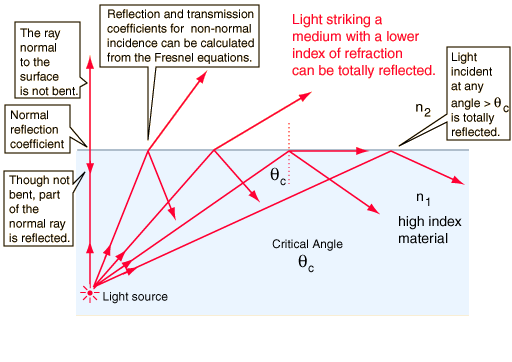
2) Total Internal Reflection(TIR)
It is a phenomenon that occurs when the incident ray is in the optically denser medium and when the angle of incidence is greater than the critical angle.
It is observed that when light undergoes TIR, all the light is reflected at the interface.
Note: The light follows the law of reflection such that the angle of incidence is equal to the angle of reflection.
3) Niels Bohr atomic model
Nield Bohr is a Danish Physicist
He proposed an idea that electrons in an atom move in shells around the nucleus.
He presented his explanation of line spectra in 1913.
He suggested that the energy of an electron is quantised.
A quantum is a tiny unit of energy, the value of which depends on the frequency.
A specified value for an electron is called its energy level.
It is of utmost importance to know the energy level of each orbital before proceeding to electronic configuration.
It tell you which orbitals comes first.
| Niels Bohr atomic model |
-----------------------------------------------------------------------------------------------------------------------
3. How are these knowledge and skills useful and relevant to the real world?
Well in the case of Total Internal Reflection, the concept can be applied to optical fibres which can be very useful in Telecommunication and Endoscopy.
For telecommunication,
Well in the case of Total Internal Reflection, the concept can be applied to optical fibres which can be very useful in Telecommunication and Endoscopy.
| Optical fibre |
A signal is first encoded on a laser beam. Light pulses are then generated and sent along an optical fibre. Many different signals can be sent down the same optical fibre simultaneously. At the end of the fibre, a receiver unit detects the light pulses, decodes them and retrieves the information sent.
An optical fibre can help to carry more information as they are thinner and thus more fibres can be bundled in to a copper wire cable of comparable diameter,
There is also less signal degradation hence fewer repeaters are needed to maintain the signal.
They are also immune to electromagnetic interference.
For endoscopy,
Optical fibre is used to collect the reflected light and carry the image to the lens at the control body.
The image can be viewed via a monitor.
If there are any black dot on the image, it indicates on of the optical fibre is broken.
----
Snell's Law can be used to identify the refractive index of an optical medium which leads to downstream application, yielding lots of benefits.
----
Whereas, for the Niels Bohr atomic model, it is for us to understand how some things work and let us understand that electrons move in shells.
Examples of how some things work:
Like the flame test where elements change the color of the flames. It is related to the atomic model of Niel's Bohr.
-----------------------------------------------------------------------------------------------
4. What I have learnt which is beyond my textbook/notes knowledge?
1) James Chadwick atomic model

----
Snell's Law can be used to identify the refractive index of an optical medium which leads to downstream application, yielding lots of benefits.
----
Whereas, for the Niels Bohr atomic model, it is for us to understand how some things work and let us understand that electrons move in shells.
Examples of how some things work:
Like the flame test where elements change the color of the flames. It is related to the atomic model of Niel's Bohr.
-----------------------------------------------------------------------------------------------
4. What I have learnt which is beyond my textbook/notes knowledge?
1) James Chadwick atomic model
In 1932, James Chadwick finally discovered the NEUTRON.
In 1930, Irene Curie and her husband have discovered that when a beam of alpha particles hit beryllium, an energetic stream of radiation is released. When this beam of radiation hits a substance rich in protons(e.g paraffin), protons were knocked loose which could be easily detected by a g\Geiger counter.

Thus in 1932, Chadwick proposed that this particle was Rutherford's neutron. He was awarded the 1935 Nobel Prize for his discovery.
This is Chadwick's equation:
| Chadwick's equation |
2) Electronic configuration (SPDF format)
There are a few principles to note first. The Aufbau principle, Pauli Exclusion principle and the Hund's rule.
The Aufbau principle states that electrons in their ground states occuply orbitals in order of increasing orbital energy level.
Pauli Exclusion principle suggest that an orbital cannot hold more than two electrons and 2 electrons occupying the same orbital must have opposite spins.
Hund's rule propose that the orbitals of a subshell must be occupied singly with parallel spins before they can be occupied in pairs.
The energy levels of subshells follow the order of spdf. S subshell has lower energy than P subshell and so on.
Further more the maximum number of electrons that can occupy each principal quantum shell(n) can be obtained by the formula 2n2.
There are also some exceptions as to the rule stated above. [E.g Cromium and Copper]
3) Successive Ionisation energy
There is a big increase in ionisation energy from one principal quantum shell to another.
The major jumps indicate the change of principal quantum shells.
The minor jump in ionisation energy shows evidence of subshells.
3) Successive Ionisation energy
There is a big increase in ionisation energy from one principal quantum shell to another.
The major jumps indicate the change of principal quantum shells.
The minor jump in ionisation energy shows evidence of subshells.
 |
| An example of successive ionisation energy graph |
There are some important trends though.
1) Decreasing first ionisation energies of elements down the group.
Why?
As the elements go down the group, the atomic radius increases due to an increase in the number of quantum shells. Therefore electrostatic forces of attraction between the nucleus and the outermost electron is weaker and less energy is required to remove this electron.
2) Increasing first ionisation energy of elements across the period.
Reason: Across the period, the nuclear charge increases and shielding effect remains relatively the same. Hence the effective nuclear charge increases and the electrostatic forces of attraction between the ionising electron and the nucleus also increases, resulting in more energy required to remove the outermost electron.
---- However there are 2 Anomalies. The small dip between Group II and III elements and a small dip between Group V and VI elements.-------
For the anomaly: small dip between Group II and III elements
This is because the p subshell is further away from the nucleus than the s subshell. Hence there is a weaker attraction between the nucleus and the outermost electron.
Thus less energy is require to remove the electrons in p than in s subshell.
For anomaly: small dip between Group V and VI elements
This is due to the fact that electrons in the P subshell are not paired. However, there are 2 electrons in Group VI that are paired in the P subshell. The inter-electronic repulsion between the paired electrons in the P subshell in the Group VI element. Thus less energy is require to remove one of these paired electrons from Group VI elements.
Reason: Across the period, the nuclear charge increases and shielding effect remains relatively the same. Hence the effective nuclear charge increases and the electrostatic forces of attraction between the ionising electron and the nucleus also increases, resulting in more energy required to remove the outermost electron.
---- However there are 2 Anomalies. The small dip between Group II and III elements and a small dip between Group V and VI elements.-------
For the anomaly: small dip between Group II and III elements
This is because the p subshell is further away from the nucleus than the s subshell. Hence there is a weaker attraction between the nucleus and the outermost electron.
Thus less energy is require to remove the electrons in p than in s subshell.
For anomaly: small dip between Group V and VI elements
This is due to the fact that electrons in the P subshell are not paired. However, there are 2 electrons in Group VI that are paired in the P subshell. The inter-electronic repulsion between the paired electrons in the P subshell in the Group VI element. Thus less energy is require to remove one of these paired electrons from Group VI elements.
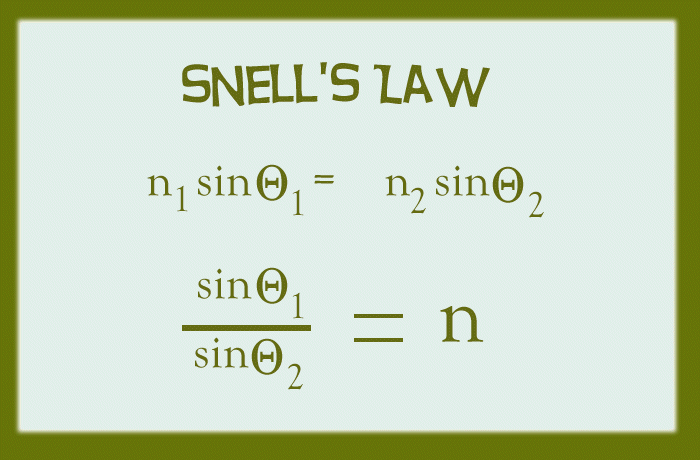
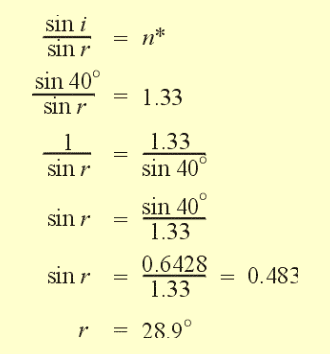
No comments:
Post a Comment